Medical Product Design.
We are an expert team of designers, engineers and strategic thinkers.
Partner with us and we will turn your product idea in to a commercial reality.
You’re likely to have questions such as is my medical product idea a novel one? What is the process involved in designing a new product? How do I go about gathering market feedback and running a clinical trial? What specialist input do I need, and how do I best navigate the Medical Device Regulations (MDR)?
ITERATE is a purpose-driven product design consultancy. Whether you are a start-up business or an established organisation, we can help get your product to market. Over the past 10 years, our team has worked with businesses across the medical; consumer; and industrial sectors. These companies have capitalised on our expert knowledge of design, innovation, engineering and manufacturing – to create new products that enhance the lives of their users and have a positive influence in the world.
We believe that product development is a vehicle for generating revenue. And, we know that major success can only be achieved if project aims are clearly defined from the start; your product is developed by following our stage-gated pathway; and most importantly, that open and honest communication remains a constant throughout.
If you choose to work with us, you will not only gain access to our design expertise but partner with a group of like-minded business people who can position you to realise a return on your investment.
Our Approach.
Our trademarked Rapid Product Development (RPD) Pathway is central to every project. Whether you want to work with us to design an award winning consumer product, or a game changing medical device – we will walk you through a series of stages so that we can achieve the best outcome for your business. Our design management approach ensures that your technical, commercial and financial risks are mitigated throughout the entire development journey.
1 - Foresight
We will determine the technical and commercial viability of your design idea at the start of your journey.

2 - Concept
Then, using digital sketching, we'll explore a variety of possibilities to establish the best design direction.

3 - Development
Our expert team will create your product in a virtual environment with specialist in-house 3D CAD software.

4 - Detail
Prototyping technologies will allow us to test your design - ensuring that it meets the needs of its users.

5 - Optimise
Final refinements will be made so that we can prepare the engineering data needed to manufacture your product.

6 - Verify
It may then be necessary to engage an external test house to verify that your product meets safety regulations.

7 - Handover
Finally, we'll provide access to our network of manufacturing partners to produce the product we have created.

Case Study.
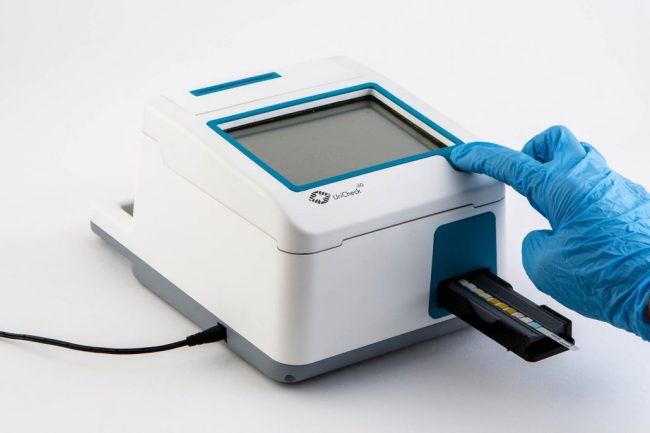
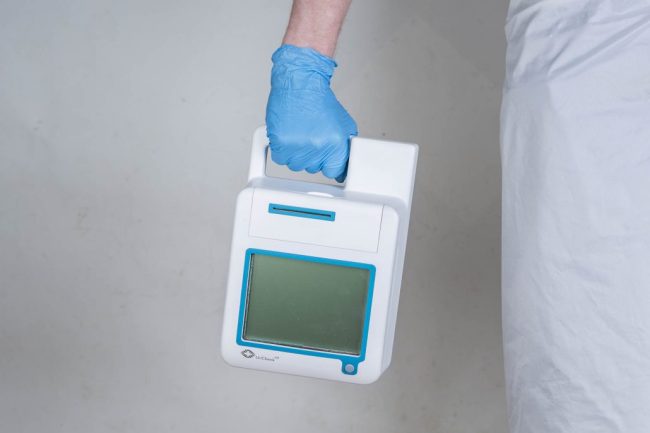
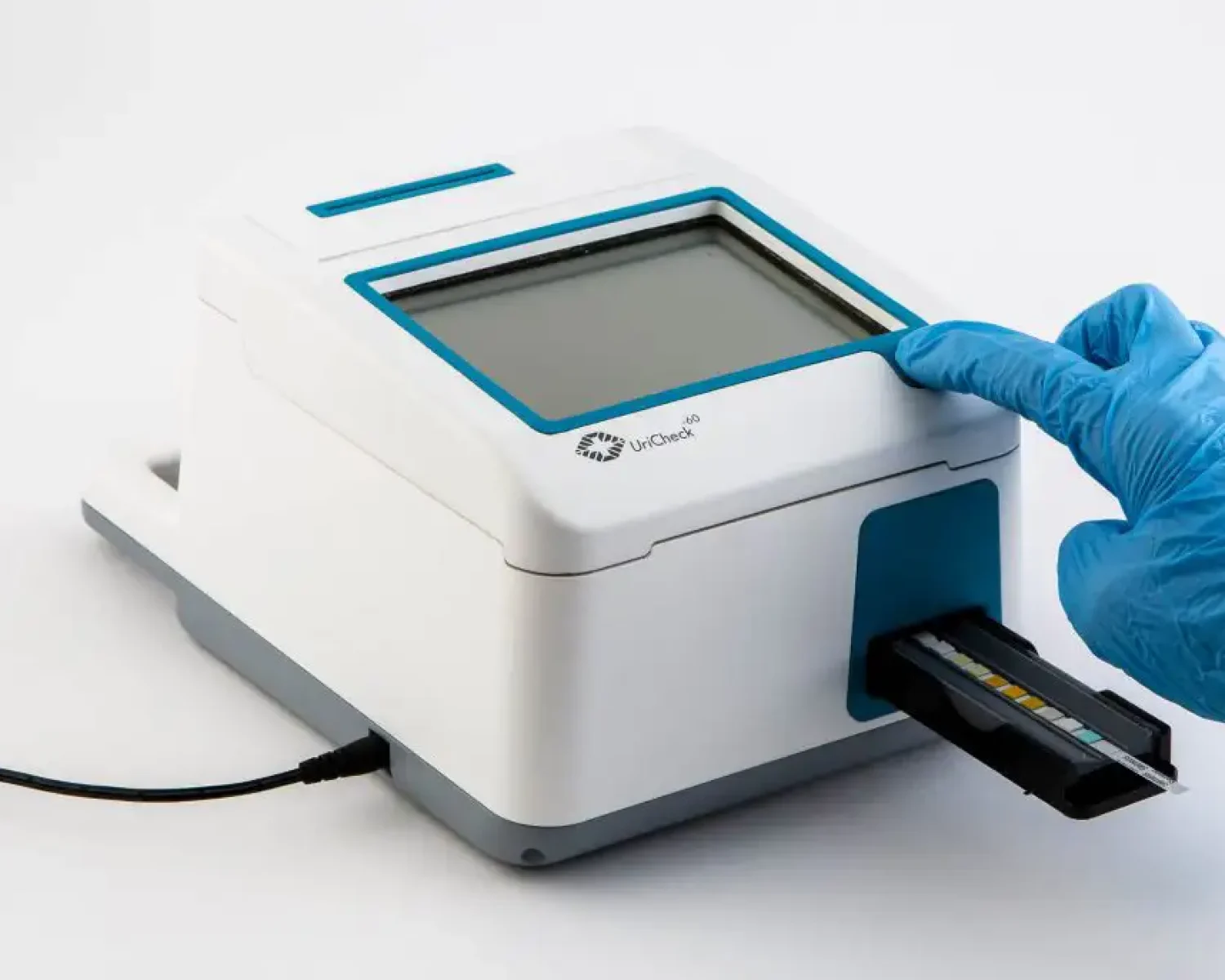
ITERATE set-about redesigning an outdated urinalysis device to demonstrate the importance of taking a user-centred approach. An observational study revealed that the usability of the diagnostic unit could be improved upon; enabling urine samples to be processed in a more streamlined way. The study also found that the time it took healthcare professionals to learn how to use the product could be drastically reduced by making the interface more intuitive. Extensive concept generation and 3D CAD modelling led to a new sleek, angular design for UriCheck, emphasising hygiene and usability. Accent colours were introduced to help highlight key user touch points such as the on/off button, graphical interface, test insertion area and printer output. A fully functional prototype was created using Stereolithography (SLA) technology and spray-painted to match the intended manufactured design.
Our Work.
We have achieved ISO 9001 & 13485 accreditation, enabling us to offer product design, research, development and
prototyping consultancy to private companies and public sector organisations in medical, consumer and industrial markets.
Customers & Partners.
“ITERATE has delivered a truly excellent prototype device for PulmonIR. I thoroughly enjoyed working with Gethin and his team and was extremely impressed with their professionalism and flexibility over this 12 month project. We are already planning for the next stage and I very much see this as a long term partnership. I am delighted to recommend Gethin and his team to anyone looking for product development support”
Dr Mark Bowman, CEO, PulmonIR Ltd.
FAQs.
How much does it cost to design a new product?
The cost of developing a new product can vary significantly depending on the product’s complexity, industry, and specific requirements. At the start of your journey, we will discuss your product idea with you in detail. This will allow us to generate a bespoke proposal – outlining each phase of work and estimated costs per phase. If your development is high risk and involves significant technical challenges to be overcome, we will recommend undertaking a package of ‘technical feasibility’ to better understand these barriers before embarking on the wider development project. It is always important to remember that launching a new product will also require investment in intellectual property, manufacturing, distribution, marketing and sales.
How long will it take to design a new product?
The time required to develop a new product varies widely depending on its complexity, scope and industry. For example, an electro-mechanical medical device may take years to properly develop; whereas, a simple consumer product may only take a few months. Based on our past experience, we are always able to offer an estimated timeline. However, it’s important to recognise that whenever trying to create a new product, challenges will occur that will need to be overcome. This is why we believe it’s important to remain flexible and communicate regularly throughout the delivery of your project.
Will ITERATE handle my design idea in confidence?
Due to the nature of our work, our team treat every design idea in absolute confidence. Even before discussing your project with us, we are able to send you a signed Non Disclosure Agreement (NDA), giving you the assurance that all commercial and technical details will remain confidential. We would also be happy to sign your company NDA document, providing the terms are mutual. Please email this directly to design@iterate-uk.com
How do I protect my design idea?
You may wish to consider protecting your design with either a patent or design registration. There are differences between these two types of intellectual property: a patent protects a novel mechanical element or process; whereas, design registration protects the overall form of a product. The costs involved in one over the other vary considerably, as well as the time needed to obtain your documentation. We would suggest discussing your IP strategy with an Attorney in our network. It is important to remember that there are many other benefits to launching a product in to the market without any protection in place but this will vary depending on your specific circumstance.
Who will own the design at the end of the project?
Each project is delivered in a number of phases and a cost is attributed against each phase of work. We request that 50% payment is made upfront and 50% payment is made on completion of each phase. Providing the work is paid for in full, you will own all intellectual property associated with a given phase of work. All design rights will automatically be transferred to you at the point of payment. If for whatever reason, you fail to pay for the work that has been undertaken, we reserve the right to retain all IP associated with your project.
Why should I partner with ITERATE to design my product?
We pride ourselves on creating not just products, but exceptional client experiences. When you choose to collaborate with us to bring your vision to life, you’re not just getting a product development partner; you’re embarking on a journey designed to prioritise your success. Our unwavering commitment to enhancing your experience sets us apart. We believe that a positive client experience is at the heart of every successful project, and we’re dedicated to making your journey with us both enjoyable and rewarding. With our team by your side, you can expect a seamless and enriching partnership, where your needs and aspirations are at the forefront of everything we do.